Background: Bispecific CD20xCD3 antibodies (BiAbs) that engage T-cells to eliminate CD20+ B-cells have emerged as a potent immunotherapy for the treatment of follicular (FL) and marginal zone (MZL) lymphomas. BiAbs have primarily been studied in patients with extensive prior immunosuppressive and lymphodepleting therapies, so their effects on the immune system without such exposures are not well understood. Unlike cellular therapies, BiAbs are administered continuously over time, spanning from 6 months to an indefinite period, and recent studies have raised concerns about T-cell exhaustion due to continuous BiAb exposure in B-lymphoblastic leukemia (Philipp et al, Blood 2022). Mosunetuzumab is a BiAb approved for treatment of relapsed/refractory FL. The ongoing phase 2 BrUOG-401 trial (NCT04792502) is investigating mosunetuzumab as first-line therapy for FL or MZL. Here, we present preliminary results focusing on treatment-induced changes in circulating immune cells.
Methods: Patients with previously untreated, high-burden or symptomatic FL or MZL received subcutaneous mosunetuzumab for 8 planned cycles (C) with step-up dosing in C1 (day 1: 5mg, day 8 & 15: 45 mg; then 45mg on day 1 of C4-C8). Patients without a complete response (CR) at interim assessment after C4 also received lenalidomide augmentation (10mg daily) during C5-8. Immune cell subsets were examined using multicolor flow cytometry (BD LSRII Analyzer). Peripheral blood samples were collected longitudinally before C1, on day 1 of C2 and C5, and 4-6 weeks after the end of therapy (EOT). Median cell abundance and marker expression were compared using generalized linear mixed models with patient and sample-level random effects. Analysis was performed in R (v.4.3.1) using the flowCore, flowStats and diffcyt packages; uncorrected P<0.05 was considered statistically significant.
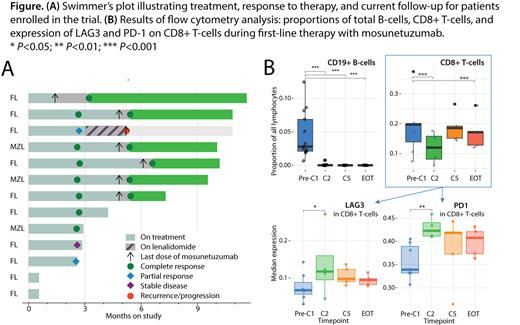
Results: As of July 2023, 13 patients (median age 62, 77% women, 77% FL, 23% MZL) were accrued in the trial. At data cutoff, 7 have completed therapy, 11 were evaluable for response, and 8 attained CR ( Fig. A). So far, only 1 patient started lenalidomide; samples collected while on lenalidomide were excluded from analysis. No instances of cytokine release syndrome (CRS) or neurotoxicity were observed. CR's occurred early (by C5). Mosunetuzumab resulted in a rapid, deep, and sustained depletion of circulating CD19+ B-cells. A significant decrease in circulating CD8+ T-cells was noted at cycle 2 (from median 20% to 12%, P<0.001; Fig. B), followed by recovery (19% at C5). Among the CD8+ subsets, the naïve CD8+ T-cells (CD45RA+CCR7+CD27+) decreased relatively at C5 & EOT, while effector memory T-cells (CD45RA-CD45RO+CD27-CD62L-) increased. Notably, we observed a significant early, temporary, increase in the expression of LAG3 at C2 on CD8+ T-cells ( P=0.033), and a more pronounced increase in PD1 expression ( P=0.001), which persisted through the EOT ( Fig. B).
No significant changes were observed in total abundance of CD4+ T-cells. However, CD4+CD25+CD127- Tregs notably increased at C2 ( P=0.008). This increase specifically driven by CD45RA+ “naïve” Tregs ( P<0.001 at C2), which remained elevated relative to CD45RO+ Tregs at C5 and EOT. All Tregs showed a significant increase in LAG3 expression at C2 ( P<0.001), which then subsided. In contrast to CD8+ T-cells, there was no significant increase in PD1 expression on CD4+ T-cells ( P=0.20 at C2). Throughout the therapy, there was a progressive increase in NK cells ( P=0.001 at EOT), with no significant shift in the relative proportions of CD16high or CD161high NK cells.
Conclusions: In this initial investigation of immune cell changes in patients who received mosunetuzumab without prior chemotherapy exposure, we observed B-cell depletion and a temporary decrease in CD8+ cytotoxic T-cells (implying their migration out of the bloodstream), aligning with mosunetuzumab's mechanism of action. Additional data from this trial will aid in understanding whether the persistent increase in PD1+CD8+ T-cells and expansion of Tregs are linked to a functionally exhausted phenotype and whether lenalidomide could potentially reverse it. Although our observation is limited by sample size, it can still provide insights into optimizing the administration schedules of BiAb therapy in future trials (e.g., intermittent vs. continuous) or determining the most suitable timing for checkpoint inhibitor combinations.
OffLabel Disclosure:
Olszewski:Leukemia & Lymphoma Society, Genetech, Inc. / F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, Genmab: Research Funding; Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, BeiGene: Consultancy. Ollila:ADC Therapeutics: Honoraria; Ono Pharmaceuticals: Honoraria, Research Funding. Huntington:Servier Pharmaceuticals LLC: Consultancy; TG Therapeutics: Consultancy; Tyme Inc: Consultancy; Seagen Inc.: Consultancy; Pharmacyclics LLC, An AbbVie Company: Consultancy; Novartis: Consultancy; Merck: Consultancy; Lilly USA, LLC: Consultancy; Janssen Pharmaceuticals: Consultancy; Genentech: Consultancy; Epizyme, Inc.: Consultancy; BeiGene USA, Inc.: Consultancy; Bayer Healthcare: Consultancy; AstraZeneca: Consultancy; Arvinas: Consultancy; ADC Therapeutics: Consultancy; AbbVie: Consultancy. Matasar:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria; Teva: Consultancy; Immunovaccine Technologies: Research Funding; Janssen: Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy; Regeneron: Honoraria, Other: Stipends; Kite: Honoraria, Other: Stipends; Immunovaccine Technologies: Honoraria; Epizyme: Other: Stipends; Celegene: Honoraria, Other: Stipends; BMS: Honoraria, Other: Stipend; Bayer: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria, Other: Stipend; Merck: Current equity holder in private company; ADC Therapeutics: Consultancy, Honoraria, Other: Stipend; Seagen: Honoraria, Other: stipends. Reagan:Pfizer: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees.
Mosunetuzumab is approved for treatment of follicular lymphoma as 3rd or subsequent line of therapy. It used in this trial as first-line therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal